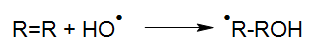uv reactors, photo-oxidation, advanced oxidation (aop), recycling & engineering
for industrial use
Chemistry of UV Oxidation and Advanced Oxidation
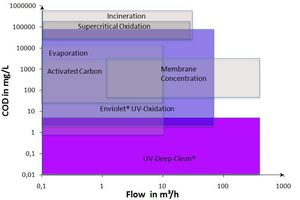
enviolet specializes in „advanced oxidation processes“ (AOP) for industrial applications. During this treatment, we differ between two different advanced oxidation process groups:
- Enviolet®: UV advanced oxidation of highly concentrated solutions in a homogeneous state (H2O2/UV process as UV-based advanced oxidation process)
- UV deep clean: Advanced UV oxidation of solutions in a heterogeneous state to obtain very low final concentrations (UV/catalyst-process)
Basics of UV Oxidation
For a photoreaction of a molecule R to take place, this molecule has to absorb light (Calvert und Pitts, 1966).
The amount of this absorbed light A(λ) is described by the Lambert-Beer law, according to which I(0) defines the light intensity prior to the absorption of light and I is the remaining light intensity after the absorption through the solution.
The light absorption depends on the concentration C of the layer thickness d and the decadal extinction coefficient ε(λ).
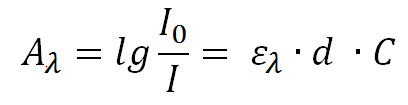
By absorbing light with sufficient energy, a molecule R* is generated from the molecule R by elevating its energetic state by the input of photon energy. Essentially this absorption is a basic requirement for any type of photoreaction. This means that even very dark solutions may initially be treated by UV oxidation if the reactors have been designed and built properly.
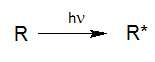
R* may return to its inital state either directly or via reactive intermediates or continue to react physically into photo products. Due to subsequent chemical processes, radicals, radical ions, ions or stable fragments are generated in the aqueous solutions, which may continue their reactions through thermal processes.
In presence of oxidizing agents additional reactions occur:
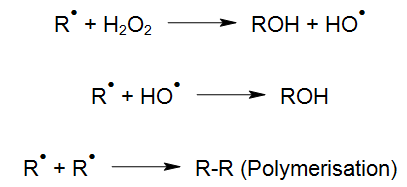
For instance, with light of appropriate wavelength, hydrogen peroxide (H2O2) can be transformed into highly reactive hydroxyl radicals by means of photolysis (UV/H2O2-process), which react quickly with organic and inorganic water compounds (LAMING et al. 1969, BAXTON and WILMARTH 1963, HOCHNADEL 1962):
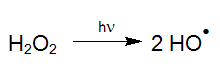
Such hydroxyl radicals (OH-radicals) are not only generated with the smallest amount of chemicals (Legrini et al. 1993) but also with the most economical energy input (Bolton and Cater 1994) by the UV/H2O2 process. Due to this, AOP is also suitable at high target concentrations to treat pollutants effectively in aqueous solutions such as, highly contaminated wastewater, electroplating baths and even ultrapure process water. For treatments at ppb-level however, catalysts are commonly used instead of H2O2 (see UV-Deep-Clean).
The degradation of organic compounds via OH radicals is initiated through hydrogen abstraction (HABER und WILLSTÄTTER 1931):
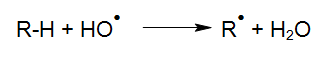
Following the initiation of these reactions, various reactions of the generated radicals become possible.
In the presence of oxygen, an organic peroxyl radical is formed:

Generally, polymerisation is not considered to be favourable reaction because polymerised products may lead to precipitation issues on the UV lamp surface. This is why it is important to avoid such polymerisation by a proper process control design and UV reactor construction. In such cases, additional process equipment may be necessary to achieve optimal reaction conditions for an efficient oxidation process.
The peroxyl radical (RHO2•), for instance, may also continue its reaction as follows:
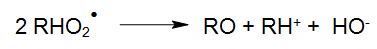
The resulting aldehydes and accordingly ketones will oxidise through the subsequent reaction processes into carboxylic acids, which are subject to either a thermal or photo-chemical decarboxylation (WEEKS und MATHESON 1955).

Typical Breakdown Products During UV-Oxidation
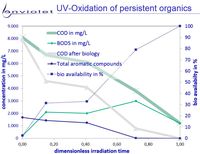
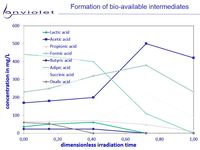
UV-Oxidation and UV-AOP of recalcitrant or toxic organics increases bio-availability of this solution in nearly all cases.
In any case BOD/COD raises from <5% to >>60%. In rare cases bio-availability is increased by small ratios only.
The picture "Formation of degradation products" it is clearly visible, that degradation products from very recalcitrant compounds are mainly small organic carbonates.
All of them are known as very good for biological degradation, and it is understood very clear that UV-Oxidation leads to good improvment of waste water quality.
Visible Change of UV Oxidation on Water Quality
Typical color changes during the treatment process of an industrial wastewater with the Enviolet® UV oxidation process are visible in the samples.